Life is built on chemistry, but the chemistry required to jump from the basic amino acids and small organic molecules expected on the Early Earth to the components of Life-As-We-Know-It – lipids, proteins and nucleic acids – has been obscure, until now. The scenario outlined in Nature Chemistry [doi:10.1038/nchem.2202] also explains why the various chemical components of our kind of life are so very similar. Even though they perform quite different roles, the building blocks are similar, produced originally by very similar chemical processes.
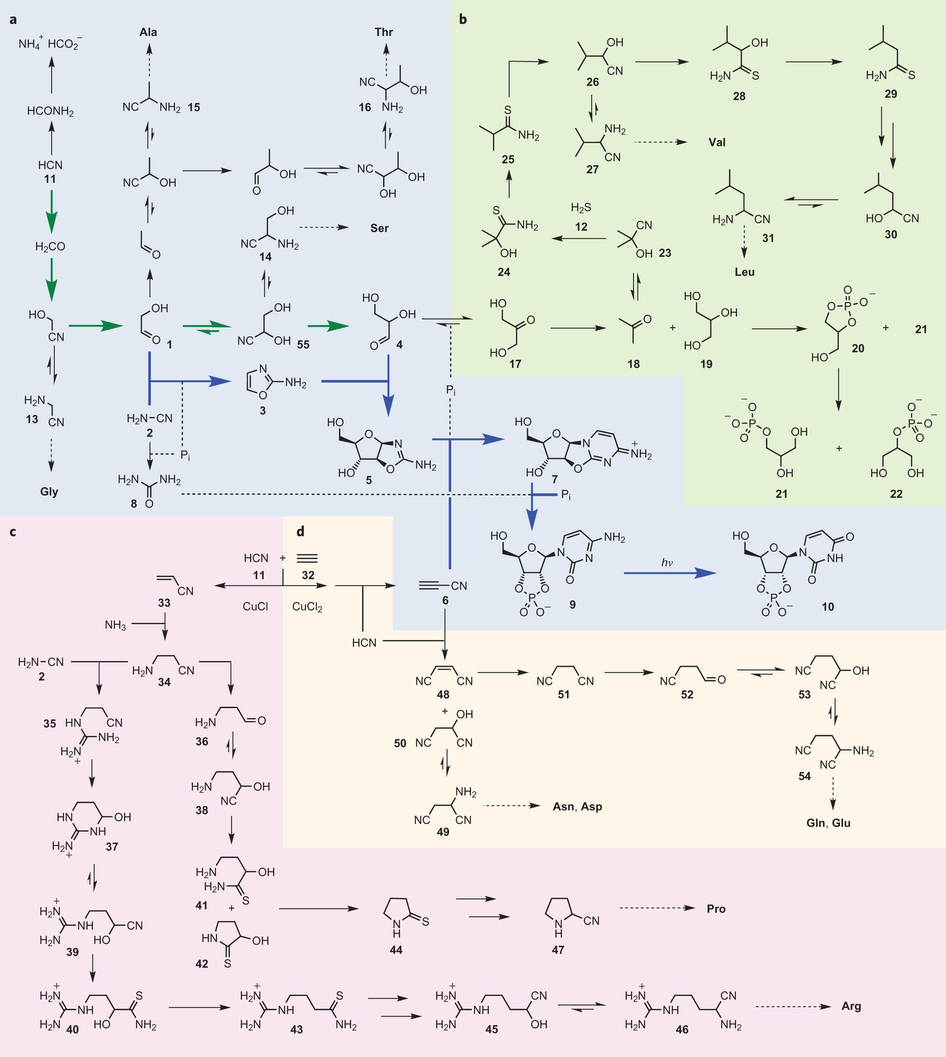
(a) Reductive homologation of hydrogen cyanide (11) (bold green arrows) provides the C2 and C3 sugars — glycolaldehyde (1) and glyceraldehyde (4)—needed for subsequent ribonucleotide assembly (bold blue arrows), but also leads to precursors of Glycine, Alanine, Serine and Threonine.
(b) Reduction of dihydroxyacetone (17) (the more stable isomer of glyceraldehyde (4)) gives two major products, acetone (18) and glycerol (19). Reductive homologation of acetone (18) leads to precursors of Valine and Leucine, whereas phosphorylation of glycerol (19) leads to the lipid precursor glycerol-1-phosphate (21).
(c) Copper(I)-catalysed cross-coupling of hydrogen cyanide (11) and acetylene (32) gives acrylonitrile (33), reductive homologation of which gives precursors of Proline and Arginine.
(d) Copper(II)-driven oxidative cross-coupling of hydrogen cyanide (11) and acetylene (32) gives cyanoacetylene (6), which serves as a precursor to Asparagine, Aspartic acid, Glutamine and Glutamic acid. Pi, inorganic phosphate.
The key-point is the relatedness and the step-by-step creation of one component or another, by short chemical processes, from the basic materials. To have produced such serial chemistry would have required means of isolating the raw materials and products, then mixing them. The next image from the paper provides a hint of what would’ve been required, on some sun-drenched landscape, swept by occasional rains, in an atmosphere of (probably) H2, N2, CO2 and H2O…
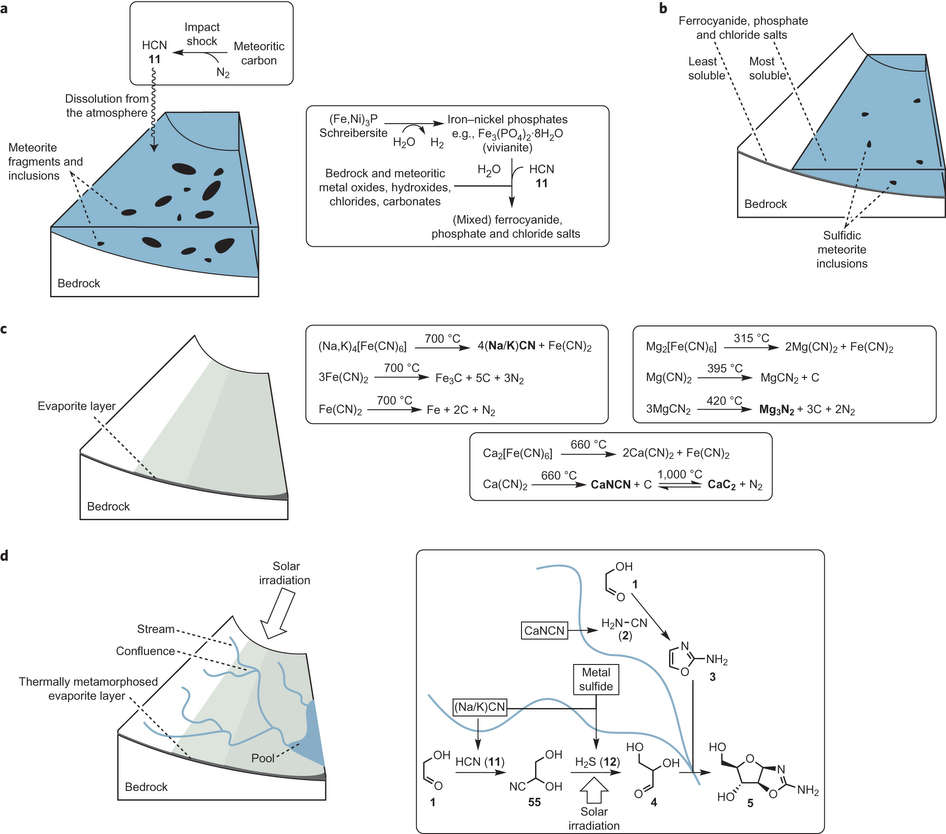
(a) Dissolution of atmospherically produced hydrogen cyanide results in the conversion of vivianite (the anoxic corrosion product of the meteoritic inclusion schreibersite) into mixed ferrocyanide salts and phosphate salts, with counter cations being provided through neutralization and ion-exchange reactions with bedrock and other meteoritic oxides and salts.
(b) Partial evaporation results in the deposition of the least-soluble salts over a wide area, and further evaporation deposits the most-soluble salts in smaller, lower-lying areas.
(c) After complete evaporation, impact or geothermal heating results in thermal metamorphosis of the evaporite layer, and the generation of feedstock precursor salts (in bold).
(d) Rainfall on higher ground (left) leads to rivulets or streams that flow downhill, sequentially leaching feedstocks from the thermally metamorphosed evaporite layer.
Solar irradiation drives photoredox chemistry in the streams. Convergent synthesis can result when streams with different reaction histories merge (right), as illustrated here for the potential synthesis of arabinose aminooxazoline (5) at the confluence of two streams that contained glycolaldehyde (1), and leached different feedstocks before merging.

One Reply to “Making the Stuff of Life in One Batch”
Comments are closed.